why does oil and water not mix
And oil and water dont have much in common. Water will not do this so the oil is forced to stay separate from the water.
 |
| Why Oil And Water Does Not Mix Together |
The attraction exists because water is a polar molecule.
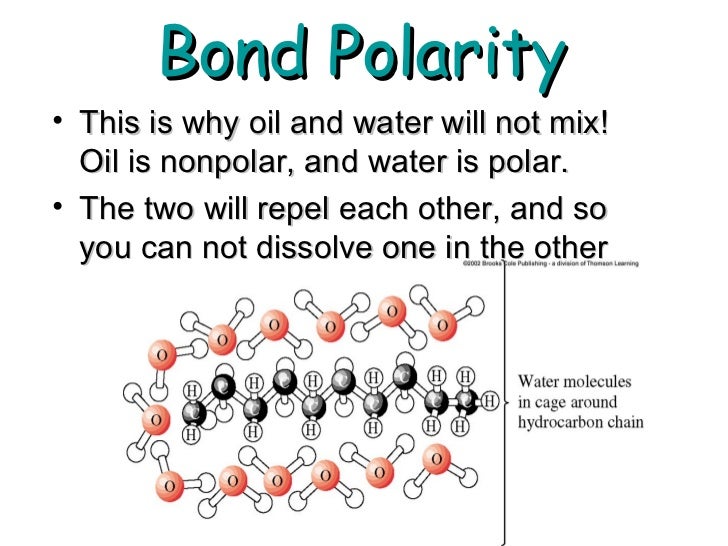
. This property comes from what are called intermolecular forces. Why water and oil dont mix. Oil is composed of the elements carbon and hydrogen. Water and oil dont mix because in the mixtures world.
Oils on the other hand are nonpolar. Liquid water is held together by hydrogen bonds. 1 2 in oil water. The low solubility is because the attraction force between water and oil molecules is weak and not able to overcome the very strong hydrogen bonding that water has with itself.
These are attractions between atomsmolecules that. They mix and dissolve in other nonpolar hydrophobic compounds. Liquid water has fewer hydrogen bonds than ice Oils and fats not have any polar part and so for them to dissolve in water they would have to break some of water s hydrogen bonds. Instead the oil slowly rises to the top of the water.
Water molecules are highly polar with an electronegative oxygen atom pulling charge away from two hydrogen atoms and. The first thing you will observe is that oil and water will not stay mixed together no matter how hard you shake the jar. Oil and water do not mix because they have different chemical compositions and as such have different physical properties. Water is a tiny polar molecule.
In fact oil floats on water because it is less dense with these super-tight hydrogen bonds between water molecules holding them closer together than the bonds between the fatty-acid. A water molecule is Polar and oil is non-Polar in nature. The other scientific reason why oil and water do not mix is their chemical nature. The basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of the clump.
In a water molecule oxygen atoms are. Like a glass of cold water on a hot summer day the colder surface causes the water vapor to precipitate. Basically because water is much more polar than oils. Oil and water do not mix Said of two objects elements factors forces people etc that do not or cannot mix together readily.
Why oil and water do not mix. Refers to the natural tendency of oil and water to separate. The condensing water then forms droplets at the surface that spread. Hydrophobic does not mix with water Hydrophilic mixes with water.
 |
| Why Do Oil And Water Not Mix Youtube |
 |
| Which Term Best Describes The Substances That Cause Oil And Water Molecules In Salad Dressing To Brainly Com |
 |
| Mixing Oil Water Science Experiment |
 |
| Fire And Emergency Nz Never Pour Water On An Oil Fire Water And Oil Do Not Mix If Your Frypan Catches Fire Do Not Throw Water On It This Can Make |
 |
| Maries Brush Cleaner 100 Ml Mo 3011 For Oil Colour Lazada Indonesia |
Posting Komentar untuk "why does oil and water not mix"